Cell Based Assays
-
Posted: January 06, 2026Read more »
Apolipoprotein E (ApoE) is a key regulator of lipid transport and cholesterol homeostasis, and genetic differences in the APOE gene significantly influence susceptibility to neurodegenerative and cardiovascular diseases. Understanding a sample’s APOE genotype provides essential biological context across
-
Posted: November 24, 2025Categories: Cell Based AssaysRead more »
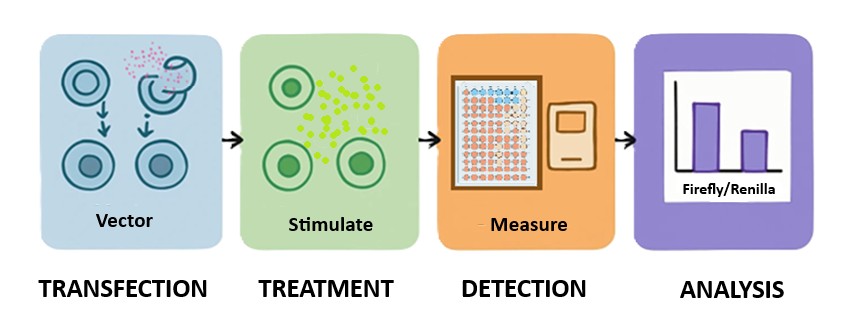
Seeing Gene Activity in Real Time
Cells constantly respond to changes in their environment—switching genes on and off in response to stress, nutrients, or signaling molecules. Studying how and when these genes are activated is essential for understanding cellular communication, disease progression, and
-
Posted: May 27, 2025Read more »
Several key metabolites play crucial roles in cellular bioenergetic homeostasis. ScienCell has developed sensitive assays to accurately quantify L-lactate, glucose, glutamate, and pyruvate.
L-Lactate is a key intermediate in glucose metabolism. Under hypoxic conditions, lactate dehydrogenase
-
Posted: May 15, 2025Read more »
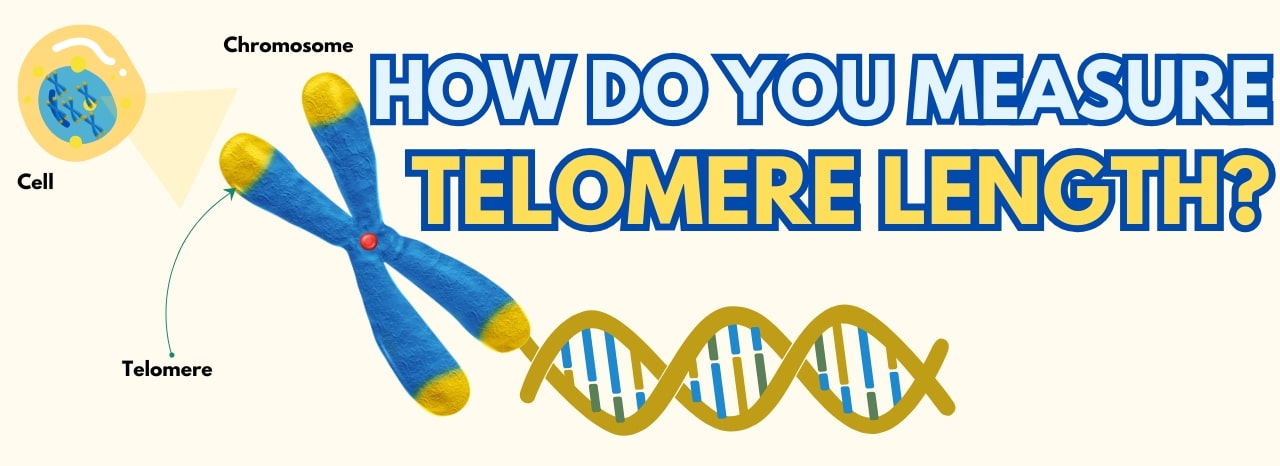
Advance Your Telomere Research Using ScienCell’s Absolute Telomere Length Quantification qPCR Kits!
Understanding Telomeres and Their Role in Genomic Stability
Telomeres are nucleoprotein structures that cap chromosome ends, protecting them from degradation and ensuring genomic stability. Each cell
-
Posted: October 06, 2022Read more »
Telomere “caps” are located at the ends of all chromosomes, including those of the immune system’s T-lymphocytes (T cells), and become shorter with every cell division. Once these chromosomes reach a point where the telomere is too short to provide adequate protection, division ceases and the cell proceeds
-
Posted: April 20, 2020Read more »
SARS-CoV-2 is the seventh known coronavirus that causes the human disease known as COVID-19. The virus can grow in cells lining the conducting airways and in alveolar epithelial cells. First, the virus generally enters the body through the nose or mouth. From there, the virus travels down into the alveoli
-
Posted: July 22, 2019Comments: 2Read more »
Traditional 2D cultures have been used widely over the past decades to study cell biology, molecular biology and conduct translation research such as drug discovery. Cells in 2D culture, however, are forced to adopt a planar morphology and maintain cellular interactions only in lateral directions, altering
-
Posted: August 19, 2018Categories: Cell Based AssaysRead more »
Cell-based assays are widely used in basic and translational research as cost-effective and accessible models to mimic in vivo responses. To obtain reliable data, assessing the health of cultured cells prior to any assays is highly recommended. Furthermore, many cell-based assays require quantification
-
Posted: November 29, 2017Read more »
Primary cells, which are isolated directly from tissue, show normal cell morphology and maintain many of the important markers and functions seen in vivo. Primary cells, though, have a finite lifespan and limited expansion capacity, so it is critical to use low passage primary cells for your research.
-
Posted: July 04, 2017Categories: Cell Based AssaysRead more »
Cell culturing is a widely used technique to grow cells outside of their natural environment using artificial environments and controlled conditions that has become indispensable to scientific research. The applications of cell culturing are innumerable and there are many ways to differentiate types
-
Posted: March 26, 2017Categories: Company, Information, Gene Expression Profiling, Cell Culture Media, Primary Cells, Cell Based Assays, News
-
Posted: December 06, 2016Read more »
Cell-based assays are widely used in basic and translational research as cost-effective and accessible models to mimic in vivo responses. To obtain reliable data, assessing the health of cultured cells prior to any assays is essential. Furthermore, many cell-based assays require quantification of cell





.jpg)






.png)
