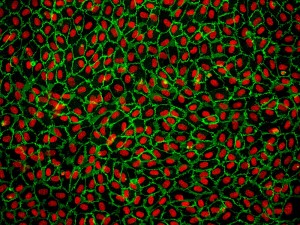
Cell culture studies provide a valuable complement to in vivo experiments, allowing for a more controlled manipulation of cellular functions and processes. For decades, cell lines have played a critical role in scientific advancements, yet researchers have become increasingly cautious when interpreting data generated from cell lines only. Factors such as misidentified and contaminated cell lines have spurred renewed interest in primary cells [1,2]. Many researchers have chosen to work with cells lines as they are generally highly proliferative, and easier to culture and transfect. Most cell lines have been in culture for decades and are well adapted to the two-dimensional culture environment, and as a result, often differ genetically and phenotypically from their tissue origin and show altered morphology [3,4]. In contrast to cell lines, primary cell which are isolated directly from tissues, have a finite lifespan and limited expansion capacity. On the positive side, primary cells have normal cell morphology and maintain many of the important markers and functions seen in vivo [3,4]. Endothelial cell lines, for example, lack various functional markers, while primary endothelial cells retain these critical features.
Primary cells, in contrast to cell lines, are extremely sensitive cells requiring additional nutrients not included in classical media. To optimize survival and growth, primary cell perform best in specialty media customized for each cell type. An endothelial cell, for example, has very different nutritional requirements than an epithelial cell or a neuron, anhttp://www.sciencellonline.com/products-services/primary-cells.htmld thus requires a unique medium. Traditional cell culture media has relied on serum to provide the growth factors, hormones, lipids and other undefined components to support cellular growth. For primary cells, however, high serum levels can lead to differentiation or promote growth of contaminating cells like fibroblasts. In addition, serum is plagued by rising costs and lot to lot variability. Formulating specialty media with little or no serum circumvents these issues while enabling greater customization to promote growth of individual primary cell types. Other practices, such as seeding primary cells on more physiologically relevant substrates rather than using synthetic polymers can significantly improve cell attachment, growth, and purity.
Although primary cells may be more difficult to work with, the data obtained from using primary cells is more relevant and reflective of the in vivo environment.
References:
[1] Alston-Roberts C, et al. (2010) “Cell line misidentification: the beginning of the end.” Nat Rev Cancer. 10(6): 441-448.
[2] Lorsch J, Collins F, Lippincott-Schwartz J. (2014) “Fixing problems with cell lines.” Science. 346 (6216): 1452-1453.
[3] Pan C, Kumar C, Bohl S, Klingmueller U, Mann M. (2009) “Comparative Proteomic Phenotyping of Cell Lines and Primary Cells to Assess Preservation of Cell Type-specific Functions.” Mol Cell Proteomics. 8(3): 443-450.
[4] Alge C, Hauck S, Priglinger S, Kampik A, Ueffing M. (2006) “Differential protein profiling of primary versus immortalized human RPE cells identifies expression patterns associated with cytoskeletal remodeling and cell survival.” J Proteome Res. 5(4): 862-878.

Please send me a complete catalog of your company for normal cell cultures, including price of each product if possible..
Armando Bartolazzi MD PhD
St. Andrea University Hospital
Via di Grottarossa 1035
00189 Rome Italy
Dear Armando,
Because you are located in Italy, we suggest you go through our distributor located there –
http://www.sciencellonline.com/distributors-by-country.html
Could you please point me towards the various cited sources for the introductory paragraph of this article?
Thank you for your comment, we have updated the citation for first paragraph. hope that helps.
ScienCell Team