Hepatic sinusoidal endothelial cells (HSEC) are fascinating cells that are uniquely adapted to their location in the liver. HSEC are found lining micro-vessels in the liver and are extremely specialized endothelial cells. Structurally and functionally they have distinctive features which include: open pores known as fenestra which form sieve plates, a lack of an organized basement membrane, expression of scavenger receptors, and performing endocytic activity. Notably, HSEC are highly permeable and play a critical role in removing bloodborne waste. To perform the endocytic function, HSEC express a vast array of scavenger receptors as well as the mannose receptor, which allows them to collect molecules from the bloodstream and transport them to the hepatocytes.
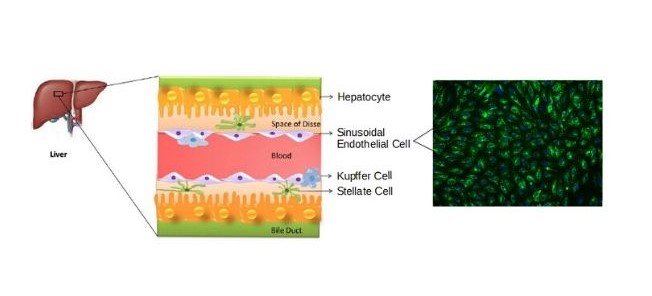
HSEC also play a pivotal role in the innate immunity by their ability to bind viruses and other pathogens through their endocytic receptors. By way of the portal vein, the liver is continuously being exposed to antigens and microbes from the gastrointestinal tract, therefore, it is important that the HSEC do not respond to all foreign bodies while also detecting and destroying harmful pathogens. To be selective, HSEC have pattern recognition receptors, such as the Toll-like receptors (TLR), which help the liver differentiate between various antigens and thereby reduce the inflammatory response. In addition to their function in the innate immune response, they also importantly regulate the adaptive immunity by antigen presentation to CD8+ T cells.
Although HSEC are vital to the immune response, their permeability may also provide an avenue for certain viruses to gain access to hepatocytes. HSEC express C-type lectin receptors, which have been associated with viral uptake. One lectin, known as liver and lymph node sinusoidal endothelial cell C-type lectin (LSECtin) interacts with Severe Acute Respiratory Syndrome (SARS) coronavirus and promotes viral infection. The role of HSEC in viral uptake of COVID-19 remains to be determined.
Studies indicate that structural modifications to HSEC can impact liver homeostasis and contribute to the development of pathologies such as hepatic fibrosis. Under normal conditions, HSEC have fenestra and lack an organized basement membrane, a state known as differentiated. Inflammation as well as other pathological factors, can cause HSEC to lose fenestrations and switch to an organized basement membrane resulting in a capillarized phenotype. This shift in phenotype from differentiated to capillarized HSEC is closely linked with the development of fibrosis. Differentiated HSEC help to maintain hepatic stellate cell quiescence, whereas, capillarized HSEC promote hepatic stellate cell activation. Consequently, hepatic stellate cell activation drives the development of fibrosis leading to liver disease.
Due to the diverse functions of HSEC, researchers are investigating the roles HSEC play in hepatic fibrosis, liver disease, immunity, and cancer. HSEC are an exciting potential target for therapeutic treatments and could be used in the future to help increase drug uptake and efficacy. ScienCell Research Laboratories is currently offering primary Human Hepatic Sinusoidal Endothelial Cells (HHSEC, Cat. #5000) for buy one, get one half off until May 14th. HHSEC are cryopreserved at passage 1 and are guaranteed for five population doublings. Additionally, we offer ready-to-use and all-inclusive versions of our 3D Human Hepatic Stellate-Endothelial Cell Spheroids (Cat. #SP3D-5000 and Cat. #3D-5000). If you are looking to perform molecular biology experiments, we also offer Human Hepatic Sinusoidal Endothelial Cell Pellets (Cat# CP5000).
Visit our COVID-19 page to learn more.
Immunostaining of the co-culture spheroids with the stellate cell markers (Vimentin and Smooth Muscle Actin) and the endothelial cell marker Von Willebrand Factor.
